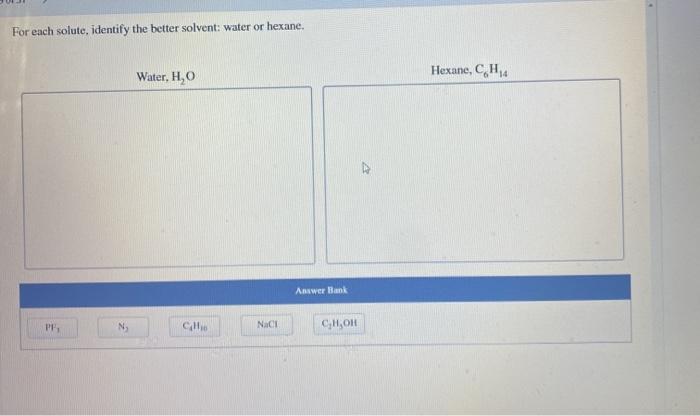
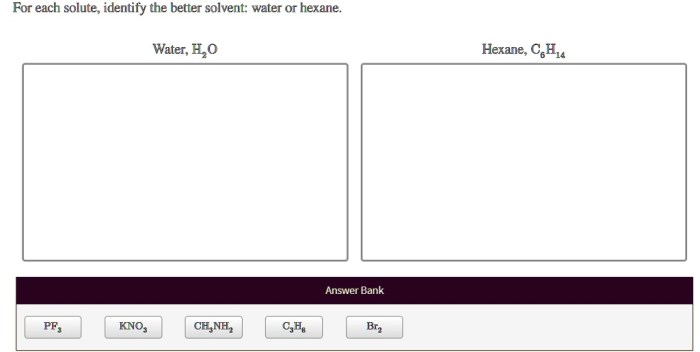
For each solute identify the better solvent water or hexane, a topic of significant importance in chemistry, explores the intricacies of solvent-solute interactions and their profound impact on solubility. By delving into the fundamental principles of polarity and hydrogen bonding, we unravel the mechanisms that govern the selection of the most suitable solvent for a given solute.
As we embark on this journey, we will discover the remarkable influence of solvent properties on solute behavior, enabling us to make informed decisions and optimize experimental outcomes. Ultimately, this understanding empowers us to harness the power of solvation for diverse applications, ranging from drug development to environmental remediation.
Solvent-Solute Interactions: For Each Solute Identify The Better Solvent Water Or Hexane
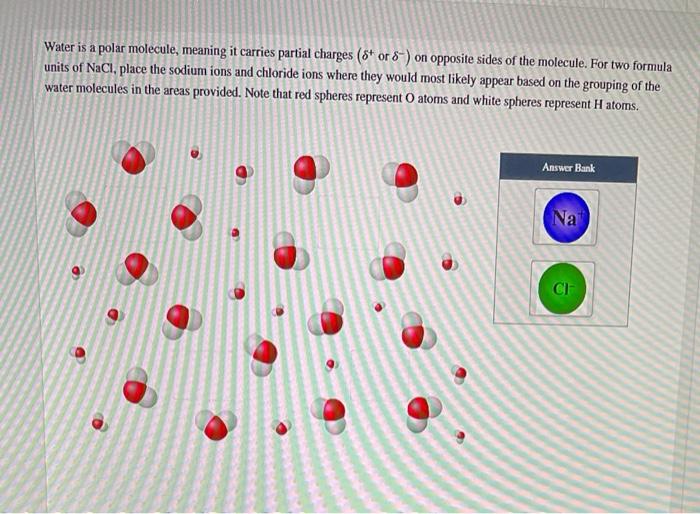
Solvent-solute interactions are the forces that determine the solubility of a solute in a solvent. These interactions can be classified into three main types: dipole-dipole interactions, hydrogen bonding, and van der Waals forces.
Dipole-Dipole Interactions
Dipole-dipole interactions occur between polar molecules that have a permanent dipole moment. A dipole moment is a measure of the polarity of a molecule, and it is determined by the difference in electronegativity between the atoms in the molecule.
The strength of a dipole-dipole interaction depends on the magnitude of the dipole moments of the molecules involved. The larger the dipole moments, the stronger the interaction.
Hydrogen Bonding
Hydrogen bonding is a special type of dipole-dipole interaction that occurs between molecules that have a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
The hydrogen atom in a hydrogen bond is partially positive, and the electronegative atom is partially negative. This creates a strong dipole-dipole interaction between the two molecules.
van der Waals Forces, For each solute identify the better solvent water or hexane
van der Waals forces are weak attractive forces that occur between all molecules, regardless of their polarity.
van der Waals forces are caused by the temporary fluctuations in the electron distribution of molecules. These fluctuations create instantaneous dipoles, which can then interact with other molecules.
The strength of van der Waals forces depends on the size and shape of the molecules involved. The larger the molecules, the stronger the van der Waals forces.
FAQ Summary
What is the significance of polarity in solvent selection?
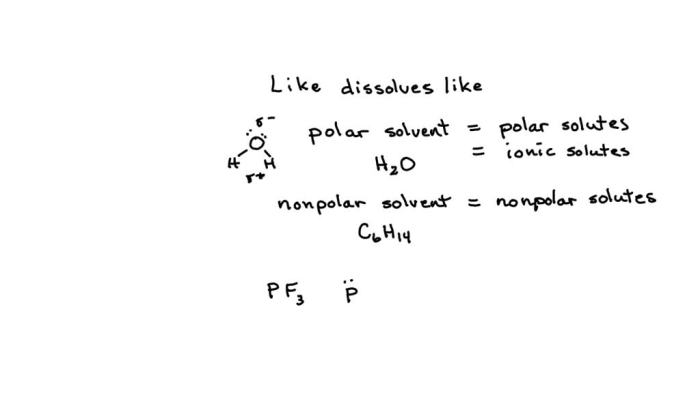
Polarity plays a crucial role in determining the compatibility between a solvent and a solute. Polar solvents, such as water, are capable of forming strong interactions with polar solutes, while nonpolar solvents, such as hexane, are more compatible with nonpolar solutes.
Understanding polarity is essential for selecting the optimal solvent for a given solute.
How does hydrogen bonding influence solvent-solute interactions?
Hydrogen bonding is a specific type of dipole-dipole interaction that occurs between molecules containing hydrogen atoms bonded to highly electronegative atoms, such as oxygen or nitrogen. Hydrogen bonding can significantly enhance the solubility of polar solutes in polar solvents, as it provides an additional attractive force between the solute and solvent molecules.
What are the limitations of using polarity as the sole criterion for solvent selection?
While polarity is a valuable guiding principle for solvent selection, it is important to note that it is not the only factor to consider. Other factors, such as steric effects and specific solute-solvent interactions, can also influence solubility. Therefore, it is often necessary to evaluate multiple solvents experimentally to determine the optimal choice for a given solute.